Beyond the "X" Factor: A Better Way to Evaluate PRP Kit Efficiency
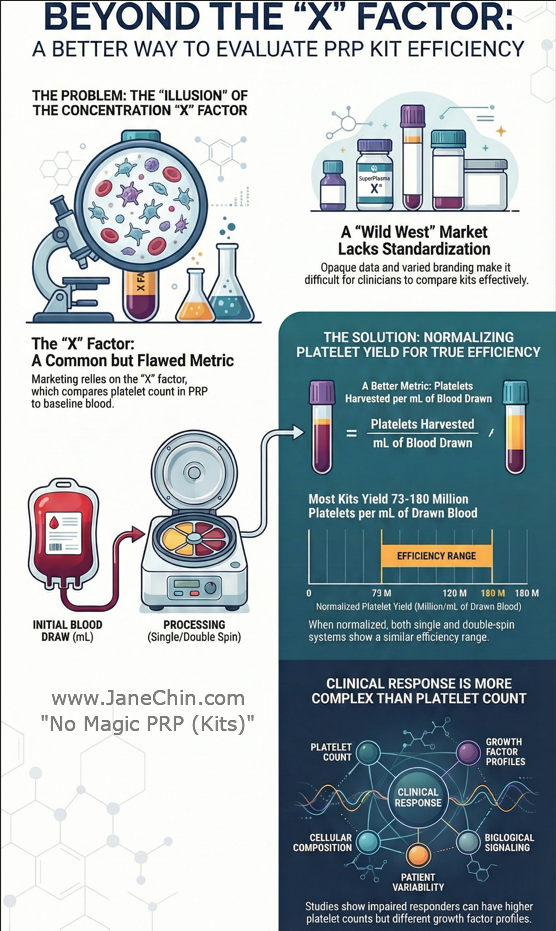
When you normalize platelet yield by blood volume drawn, the marketing illusion of "5X" or "12X" concentration multipliers disappears.
Full Research Analysis: No Magic PRP (2025)
Click here to open the PDF in a new tab
🔬 THE FUNDAMENTAL FINDING
Regardless of single- or double-spin systems, FDA-cleared PRP kits yield 73–180 million platelets per mL of drawn blood (mean: 125.5 ± 53.1). This analysis reviewed data from 6 major commercial systems to expose the marketing illusion of the concentration multiplier.
The Problem: The Illusion of the "X" Factor
Marketing for PRP systems relies heavily on concentration factors like "9X" or "12X baseline." However, this metric primarily reflects how much plasma is discarded, not the efficiency of the device in capturing platelets. Normalizing platelet yield with blood-draw volume is the missing link in demystifying these claims.
Clinical Response Is More Complex Than Platelet Count
The assumption that "more platelets equals better outcomes" oversimplifies biological reality. A recent retrospective analysis of 75 knee OA patients receiving a single injection of PRP found that impaired responders actually had significantly higher platelet counts than responders.
| Measure | Responders | Impaired Responders | Effect |
|---|---|---|---|
| Platelet Count | Lower | Significantly Higher | ↑ Paradoxical Impairment |
| VEGF Concentration | Baseline | 2× Higher | ↑ Pro-angiogenic |
| EGF Concentration | Baseline | 33% Higher | ↑ Pro-proliferative |
| TGF-β1 Profile | Optimized Balance | Altered Signaling | Context-Dependent Change |
Growth Factor Interactions
Growth factors do not act in isolation. TGF-β1 is a potent morphogenesis factor that can stimulate or inhibit cell proliferation depending on the cross-signaling with VEGF and EGF. High platelet counts in impaired responders often correlate with growth factor ratios that drive angiogenesis or apoptosis rather than healing.
⚠️ REGULATORY REMINDER
FDA-cleared PRP systems are approved for "mixing with bone graft materials to enhance handling characteristics"—not for direct therapeutic injection into joints or tendons. Off-label use requires informed consent acknowledging the lack of specific FDA review for those indications.
Key Recommendations
- Ignore "X" factor claims: They create a false sense of technological superiority.
- Prioritize response analysis: Compare "responders" to "non-responders" in your specific patient population.
- Demand transparent data: Request total platelet yield per mL of drawn blood from your distributor.
Infographic: Evaluating PRP kit efficiency through normalization of platelet yield.
References
- "Classify Your Medical Device", U.S. Food & Drug Administration.
- "Premarket Notifications 510(k)", U.S. Food & Drug Administration.
- "Product Classification: Device Platelet And Plasma Separator For Bone Graft Handling." U.S. Food & Drug Administration.
- Kon E, Di Matteo B, et al. Platelet-rich plasma for the treatment of knee osteoarthritis: an expert opinion and proposal for a novel classification and coding system. Expert Opin Biol Ther. 2020 Dec;20(12):1447-1460.
- Magalon J, Brandin T, et al. Technical and biological review of authorized medical devices for platelets-rich plasma preparation in the field of regenerative medicine. Platelets. 2021 Feb 17;32(2):200-208.
- Marín Fermín T, Scarlat MM, Laupheimer MW. Would you have an injection without knowing its formula? New challenges in platelet-rich plasma therapy. Int Orthop. 2022 Oct;46(10):2179-2180.
- Mazzocca AD, McCarthy MB, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012 Feb 15;94(4):308-16.
- Magalon J, Bausset O, et al. Characterization and comparison of 5 platelet-rich plasma preparations in a single-donor model. Arthroscopy. 2014 May;30(5):629-38.
- Oh JH, Kim W, Park KU, Roh YH. Comparison of the Cellular Composition and Cytokine-Release Kinetics of Various Platelet-Rich Plasma Preparations. Am J Sports Med. 2015 Dec;43(12):3062-70.
- Fitzpatrick J, Bulsara MK, et al. Analysis of Platelet-Rich Plasma Extraction: Variations in Platelet and Blood Components Between 4 Common Commercial Kits. Orthop J Sports Med. 2017 Jan 3;5(1).
- Kaux JF, Le Goff C, et al. Comparative study of five techniques of preparation of platelet-rich plasma. Pathol Biol (Paris). 2011 Jun;59(3):157-60.
- Atashi F, Jaconi ME, Pittet-Cuénod B, Modarressi A. Autologous platelet-rich plasma: a biological supplement to enhance adipose-derived mesenchymal stem cell expansion. Tissue Eng Part C Methods. 2015 Mar;21(3):253-62.
- Mandle RJ. Comparison of EmCyte GS30- PurePRP® II, EmCyte GS60- PurePRP® II, Arteriocyte MAGELLAN, Stryker REGENKIT®THT, and ECLIPSE PRP. BioSciences Research Associates, Inc; 2016.
- Bec C, Rousset A, et al. A Retrospective Analysis of Characteristic Features of Responders and Impaired Patients to a Single Injection of Pure Platelet-Rich Plasma in Knee Osteoarthritis. J Clin Med. 2021 Apr 17;10(8):1748.
- van Cruijsen H, Giaccone G, Hoekman K. Epidermal growth factor receptor and angiogenesis: Opportunities for combined anticancer strategies. Int J Cancer. 2005 Dec 20;117(6):883-8.
- Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997 Sep;8(3):171-9.
- Roberts AB, Sporn MB. Regulation of endothelial cell growth, architecture, and matrix synthesis by TGF-beta. Am Rev Respir Dis. 1989 Oct;140(4):1126-8.
- Hartsough MT, Mulder KM. Transforming growth factor-beta signaling in epithelial cells. Pharmacol Ther. 1997;75(1):21-41.
- Ferrari G, Cook BD, et al. Transforming growth factor-beta 1 (TGF-beta1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J Cell Physiol. 2009 May;219(2):449-58.
- Ferrari G, Pintucci G, et al. VEGF, a prosurvival factor, acts in concert with TGF-beta1 to induce endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2006 Nov 14;103(46):17260-5.
- Mu Y, Gudey SK, Landström M. Non-Smad signaling pathways. Cell Tissue Res. 2012 Jan;347(1):11-20.
- Wilson SE. Interleukin-1 and Transforming Growth Factor Beta: Commonly Opposing, but Sometimes Supporting, Master Regulators of the Corneal Wound Healing Response. Invest Ophthalmol Vis Sci. 2021 Apr 1;62(4):8.